I have been contemplating taming wild yeast. What I mean is I want to isolate clonal strains of wild yeast, be it S. cerevisiae or Brettanomyces species. By far the latter is more intriguing, as there are hundreds of S. cerevisiae strains available. To begin taming Brett stains I need two things: a source and a medium to specifically select Brett versus S. cerevisiae. Well, maybe a third thing – patience, as this is going to get tedious.
|
MYPG
|
modified
|
|
0.3% malt extract
|
0.3% malt extract
|
|
0.3% yeast extract
|
0.25% yeast extract
|
|
1% dextrose
|
1% dextrose
|
|
0.5% peptone
|
0.5% peptone
|
|
1.5% agar
|
1.5% agar
|
205-213, 1998) was 195 ppm. Chad Yakobson at brettanomycesproject.com used 1200 ppm, and Jason at sciencebrewer.com used 312 ppm. I decided to try two concentrations to see what grows – 195 ppm and 390 ppm. My stock solution of copper sulfate was 1.95% w/v CuSO4·5H2O dissolved in water, so I added 0 μl, 250μl (195 ppm), or 500 μl (390 ppm) of copper sulfate solution to 25 ml of melted agar per plate.
My control for S. cerevisiae inhibition was Wyeast 3522, and my test yeast mixture was the dregs of Jolly Pumpkin Bam Biere. I chose Wyeast 3522 (WLP550) because that is the primary yeast strain used to ferment Bam Biere.
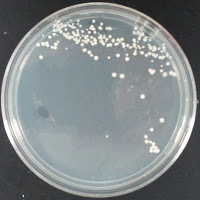 3522 0 ppm CuSO4 |
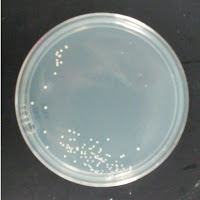 3522 195 ppm CuSO4 |
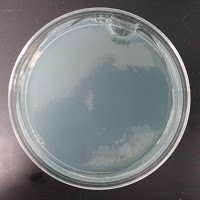 3522 390 ppm CuSO4 |
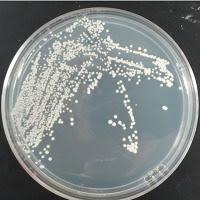 Jolly Pumpkin 0 ppm CuSO4 |
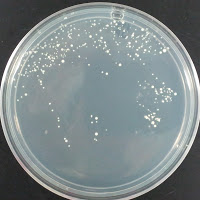 Jolly Pumpkin 195 ppm CuSO4 |
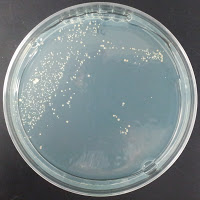 Jolly Pumpkin 390 ppm CuSO4 |
There were no colonies on the 390 ppm plates. I kept the latter plates at 30°C for several more days, and finally something (s) grew on the Jolly Pumpkin plate, but not the Wyeast 3522 plate. I was concerned that what grew out was bacterial in origin, as there is no antibiotic on the plate to suppress bacteria. I picked a couple Jolly Pumpkin colonies, and analyzed them under the microscope, and they were indeed yeast, not bacteria (not shown).
So are you going with the 390 ppm CuSO4 and have you done any yeast wrangling yet?
I have done any further work with Brett isolation – I’m been busy with my eubayanus work. However, when I do get back into wild Brett culturing, I will definitely use 390 ppm. Actually will probably just round up to 400 ppm for simplicities sake.